half life formula chemistry
Half life formula is. We can express the population after n têthalf half lives as Nn N0 -nlnH2LN 0 -t lnH2Lêthalf N 0 -069 têthalf.

Gibbs Free Energy Equilibrium Constant Enthalpy Entropy Equations Free Energy Free Energy Generator Free Energy Projects
The general equation with half life N t N 0 05 t T In which N 0 is the number of atoms you start with and N t the number of atoms left after a certain time t for a nuclide with a half life of T.

. T 12 ln2λ 0693 λ t 12 The half-life of the substance λ The disintegration constant or decay constant. Now we have the formula for the half-life of a substance. Log 1 2 N t N.
I-131 used in thyroid scans has a half-life of 802 days. The half-life of fluorine-20 is 110 s. We can describe exponential decay by the following given decay equation.
N 0 initial quantity of the substance. Using the same procedure as above you will get the expression Nn N0 10-nlogH2LN 0 10-030 têthalf. Here we identify the initial amount as 500 g t 600 s and t 12 110 s.
00000402 g Radioactive decay is an exponential process not a linear process. Science Chemistry QA Library The half-life of a certain element is about 5600 years. The half-life of a first-order reactant relates to the rate constant k using the following equation.
A piece of cloth is painted with organic dyes. Kinetics we can determine the half-life formula is t 12 ln 2k where t 12 is. The measurement of this quantity may take place in grams moles number of atoms etc.
The formula for half-life in chemistry depends on the order of the reaction. The half-life of a second-order reaction can be calculated after being given the initial concentration of the reactant and the rate constant. N t N 0 1 2 t t 1 2.
Half-life symbol t 12 is the time required for a quantity to reduce to half of its initial valueThe term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable atoms survive. 31 10 8 g. N t N 0 1 2 t t 1 2.
Use the radiometric dating formula to complete parts a and b below. For numerical calculations without calculator it is more convenient to work with base-10 logarithms. Substituting into the equation.
Half-life means the amount of time that some element takes for half of its particular sample to react. Answers Only radioactive isotopes have a half-life. What is the half-life formula.
During the next 3 years 125 grams would remain and so on. For the first-order reaction the half-life is defined as t12 0693k And for the second-order reaction the formula for the half-life of the reaction is given by 1k R 0 Where t12 is the half-life of a certain reaction unit - seconds R0 is the initial reactant concentration unit - molL-1. You can replace the N with the activity Becquerel or a dose rate of a substance as long as you use the same units for N t and N 0.
T12 is the half-life τ is the mean lifetime λ is the decay constant If an archaeologist found a fossil sample that contained 25 carbon-14 in comparison to a living sample the time of the fossil samples death could be determined by rearranging equation 1 since Nt N0 and t12 are known. To determine half-life dividing equation 1 by 2 t 12 A 0 2 t ------ 2 From equation 2 it can be seen that a zero order reaction states that the half-life depends on rate constant and the amount of initial concentration. T ½ 1 k A o Top Determining a Half Life To determine a half life t ½ the time required for the initial concentration of a reactant to be reduced to one-half its initial value we need to know.
Half-life or t½ is the time that elapses before the concentration of a reactant is reduced to half its initial value. Half-life and rate constant. Now we plug in.
By analyzing the dye in the cloth it is found that only 66 of the certain element in the dye remains. Show that this expression is correct. Half Life Equation - 14 images - half life calculations radioactive decay youtube ppt radioactive decay kinetics powerpoint presentation an easy equation to calculate the half life of an isotope c 3 calculating the decay constant sl youtube.
Half-life t 1 2 t log 2 log N o N t 2 Where N o Initial mass of the substance N t Quantity os the substance remaining t Time elapsed t 1 2 Half life of the substance Example 1 Calculate the half-life of Gold-198 given that 3257 mg of this radioactive isotope decayed to 102 mg in 135 days. T 12 half life of the substance. For example the medical.
510 d 25 y 0000170 g. For a zero order reaction the formula is t½ Ao 2k. This means that the fossil is 11460 years old.
Information on the half-life of an isotope can be used to calculate how much radioactivity of that isotope will be present after a certain period of time. There is a formula that allows calculation at any time after the initial count but we are just going to. For example if the half-life of a 500 gram sample is 3 years then in 3 years only 25 grams would remain.
If 423 10 6 g of carbon-11 is left in the body after 400 h what mass of carbon-11 was present initially. Half Life is ALWAYS first order By applying the first order integrated rate law to this described in Chapter 12. The half-life of carbon-11 is 203 min.
If a sample initially contains 500 g of fluorine-20 how much remains after 600 s. Half Life Formula One can describe exponential decay by any of the three formulas N t N0 N t N0 N t N0 Where N0 refers to the initial quantity of the substance that will decay. Half life time ln 2 ln beginning amount ending amount half life 11 69315 ln 32604 126 half life 15870 ln 25876 half life 76247 95073 half life 80198 days 4 Sodium 24 has a half life of 1496 hours.
Although similar to Example 3 the amount of time is not an exact multiple of a half-life. The term is also used more generally to characterize any type of exponential or non-exponential decay. N t mass of radioactive material at time interval t N 0 mass of the original amount of radioactive material k decay constant t time interval t 12 for the half-life.
N t quantity of the substance remaining. The rate constant k for the reaction or enough information to determine it. This means that once you know the half-life of a first-order reactant you can easily find k.
The order of the reaction or enough information to determine it. For a first order reaction t½ 0693 k and for a second order reaction t½ 1 k Ao.

Condensing Logs Logarithmic Functions Functions Math Organic Chemistry Study

As Level Physics Formula Sheet Physics And Mathematics Physics Formulas Physics

All In One Mindblowing Greatminds Science Modern Physics Science Facts Physics And Mathematics

Half Life Formulla Half Life Life Radioactive

Chemical Kinetics And Half Life Online College Chemistry Courses Chemical Kinetics Study Chemistry Half Life

Half Lives Chemistry Classroom Teaching Chemistry Chemistry Experiments

Trigonometry Poster Half Angle Identities Poster Zazzle Com Trigonometry Studying Math Learning Mathematics

Ch 5 5 Multiple Angle And Product To Sum Formulas Ppt Download Free Math Help Word Problem Worksheets Trigonometry

Van T Hoff Equation Physical Chemistry Chemical Equation Secondary Science

Suvat Rearranged Traditional Suvat Equations Are Highlighted Yellow Copy This To Your Cheat Sheet Learn Physics Physics Formulas Math Strategies

Radioactive Decay Formula Radioactive Half Life 0 693 Radioactive Decay Constant Physics Topics Science Themes Calculus

Radioactive Decay And Half Life Chemistry Lessons Chemistry Classroom Chemistry Lesson Plans

Combined Gas Law Definition Formula Examples Ideal Gas Law Boyle S Law Physical Chemistry

Calculation Of Half Life Of Radioactive Substances Half Life Physics Formulas Life
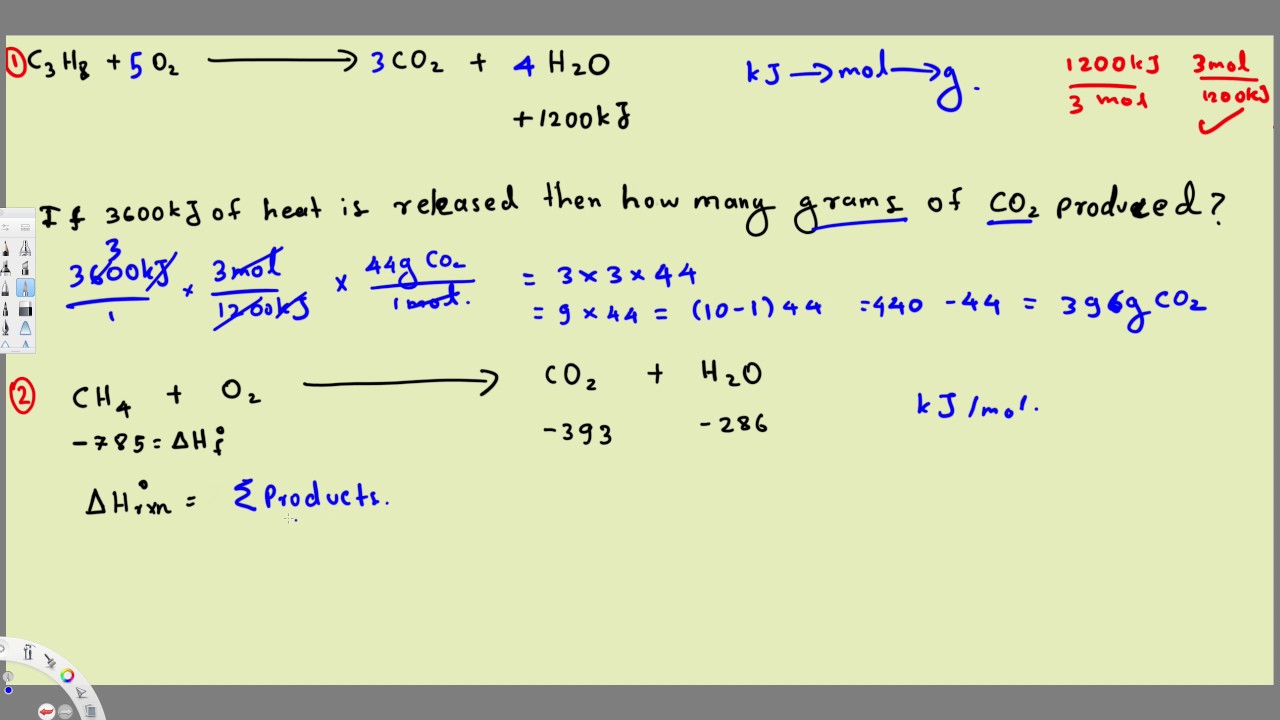
Thermochemistry Equations Formulas Practice Problems Example 2 Equations Chemistry Practice

Zero Order Kinetics Reactions Online College Chemistry Courses College Chemistry Online Chemistry Courses Chemical Changes

Half Lives Chemistry Lessons Teaching Chemistry Chemistry Experiments

Half Life Calculator Half Life Life Elapsed Time

How To Calculate The Number Of Stereoisomers A Quick Tip Chemistry Basics Chemistry Study Guide Chemistry Paper